Metastatic colorectal cancer (mCRC) continues to pose a clinical and commercial challenge, with low 5-year survival rates and limited durable responses to existing therapies. While standard chemotherapy and biologics (e.g., bevacizumab, cetuximab) have extended survival modestly, the market is shifting toward biomarker-driven treatments, particularly for patients with RAS wild-type or MSI-H tumors. Recent advances in next-generation sequencing and liquid biopsy are enhancing patient stratification, improving trial design, and informing targeted therapy development. Novel modalities—such as small molecules, antibodies, and tumor microenvironment modulators—are gaining traction and could reshape the mCRC treatment landscape over the next decade.
New York, USA, May 13, 2025 (GLOBE NEWSWIRE) — Metastatic Colorectal Cancer Clinical Trial Pipeline Accelerates as 150+ Pharma Companies Rigorously Develop Drugs for Market Entry | DelveInsight
Metastatic colorectal cancer (mCRC) continues to pose a clinical and commercial challenge, with low 5-year survival rates and limited durable responses to existing therapies. While standard chemotherapy and biologics (e.g., bevacizumab, cetuximab) have extended survival modestly, the market is shifting toward biomarker-driven treatments, particularly for patients with RAS wild-type or MSI-H tumors. Recent advances in next-generation sequencing and liquid biopsy are enhancing patient stratification, improving trial design, and informing targeted therapy development. Novel modalities—such as small molecules, antibodies, and tumor microenvironment modulators—are gaining traction and could reshape the mCRC treatment landscape over the next decade.
DelveInsight’s ‘Metastatic Colorectal Cancer Pipeline Insight 2025‘ report provides comprehensive global coverage of pipeline metastatic colorectal cancer therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the metastatic colorectal cancer pipeline domain.
Key Takeaways from the Metastatic Colorectal Cancer Pipeline Report
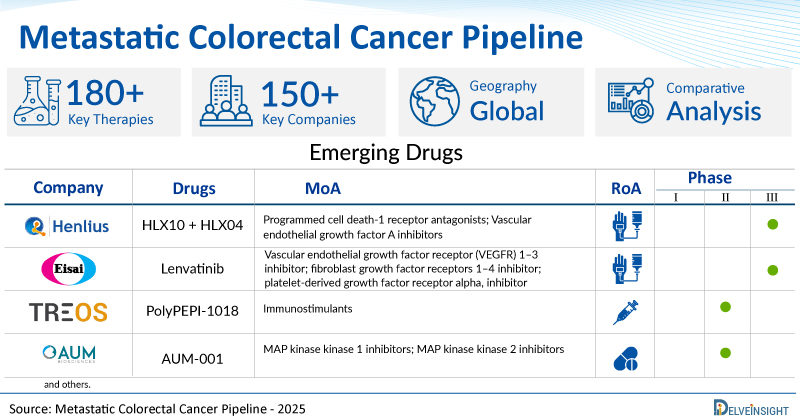
- DelveInsight’s metastatic colorectal cancer pipeline report depicts a robust space with 150+ active players working to develop 180+ pipeline metastatic colorectal cancer drugs.
- Key metastatic colorectal cancer companies such as Shanghai Henlius Biotech, Eisai Inc., Treos Bio, AUM Biosciences, Leap Therapeutics, Inc., Hoffmann-La Roche, Replimune, Qilu Pharmaceutical, Lutris Pharma, Ipsen, Bold Therapeutics, Pacylex Pharmaceuticals, FivepHusion, SystImmune, Inspirna, Inc., Sapience Therapeutics, Exelixis, Daiichi Sankyo, Hutchmed, Merus N.V., REVOLUTION Medicines, Pure Tech Health, Medicenna Therapeutics, Inc., Verastem Inc., Next Cure Inc., Isofol Medical, Zentalis Pharmaceuticals, Oncoinvent, KaryoPharm Therapeutics, Aadi Biosciences, and others are evaluating new metastatic colorectal cancer drugs to improve the treatment landscape.
- Promising pipeline metastatic colorectal cancer therapies such as HLX10 (Serplulimab) + HLX04, Lenvatinib, PolyPEPI-1018, AUM-001, DKN-01, RO7122290, E7386, RP3, QL 1706, LUT014, Liposomal irinotecan, BOLD-100, Zelenirstat, Deflexifol, SI-B003, Zanzalintinib, RGX202, ST316, Trastuzumab Deruxtecan, Surufatinib, Petosemtamab, RMC-6236, MDNA11, VS6766, NC410, LYT-200, Arfolitixorin, ZN-c3, Radspherin, KPT-8602, ABI-009, and others are in different phases of Metastatic Colorectal Cancer clinical trials.
- On March 27, 2025, LIXTE Biotechnology Holdings provided an update on its recent activities. The Company announced that the first patient had been dosed in a new clinical trial conducted in collaboration with the Netherlands Cancer Institute (NKI) and supported by F. Hoffmann-La Roche Ltd. (“Roche”) for the treatment of unresponsive (MSI Low) metastatic colorectal cancer.
- On January 25, 2025, Exelixis announced results from an expansion cohort of the Phase Ib/II STELLAR-001 trial that evaluated zanzalintinib alone or in combination with atezolizumab (Tecentriq) in patients with previously treated metastatic colorectal cancer. The findings were presented during Poster Session C: Cancers of the Colon, Rectum and Anus, at 7:00 a.m. PT on January 25 at the American Society of Clinical Oncology 2025 Gastrointestinal Cancers Symposium (ASCO GI 2025).
- In January 2025, Marengo Therapeutics announced that the US Food and Drug Administration (FDA) has granted Fast Track designation (FTD) to invikafusp alfa (STAR0602), Marengo’s first-in-class selective dual T cell agonist being studied as a potential new treatment for advanced colorectal cancer with TMB-H.
- In January 2025, Mirror Biologics announced that it had entered into a clinical trial collaboration and supply agreement with Merck KGaA, Darmstadt, Germany. The parties will collaborate to conduct a Phase II clinical trial to evaluate the combination of an experimental immunotherapy drug, AlloStim from Mirror, and an immune checkpoint inhibitor (ICI), avelumab (BAVENCIO) from Merck KGaA, Darmstadt, Germany, in fourth-line metastatic colorectal cancer.
- In December 2024, Tanner Pharma, announced a collaboration with Agenus to provide expanded access to botensilimab (BOT) and balstilimab (BAL). Through a Named Patient Program (NPP), this initiative offers patients with microsatellite stable colorectal cancer (MSS CRC) and other advanced solid tumors the opportunity to access BOT/BAL based on supporting clinical evidence and medical need. Tanner Pharma will manage access to BOT/BAL for patients in geographies that allow named patient access to investigational medicines.
Request a sample and discover the recent advances in metastatic colorectal cancer drugs @ Metastatic Colorectal Cancer Pipeline Report
The metastatic colorectal cancer pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage metastatic colorectal cancer drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the metastatic colorectal cancer clinical trial landscape.
Metastatic Colorectal Cancer Overview
Colorectal cancer (CRC) ranks as the third most prevalent cancer worldwide, with metastasis being the leading cause of mortality among affected individuals. The liver and peritoneum are among the most common sites where the cancer spreads. CRC originates in either the colon or rectum and is often referred to as colon or rectal cancer based on the location, though the two are frequently grouped together due to their shared characteristics. The disease typically begins when polyps—small, mushroom-like growths in the colon—become cancerous or when genetic mutations in the lining cells of the colon or rectum lead to uncontrolled cell growth, forming tumors.
Initially, CRC starts as a benign polyp in the innermost layer of the colon or rectum. Over time, this polyp can transform into cancer, invading deeper layers and potentially spreading through blood or lymphatic vessels. Once it enters the bloodstream or lymph system, cancer cells may travel to nearby lymph nodes or distant organs. The liver, lungs, and peritoneum are the most frequent sites of metastasis, though the disease can also reach areas such as the brain and bones.
Once CRC is diagnosed, staging is performed to determine the extent of spread. The cancer stage indicates how far the disease has progressed and helps guide treatment planning. Staging ranges from stage 0 (earliest, non-invasive) to stage IV (advanced, widespread). Generally, a lower stage implies a more localized disease with a better prognosis, while higher stages represent more advanced cancer with greater spread. Although each patient’s journey is unique, cancers at the same stage often have similar outcomes and treatment strategies.
For many patients, metastatic colorectal cancer remains incurable. The liver, lungs, lymph nodes, and peritoneum are typical sites of metastasis. About 15 years ago, the median overall survival (mOS) for metastatic CRC was roughly 12 months, with only 13% surviving beyond five years. Thanks to advancements in both surgery and systemic therapies, survival outcomes have improved significantly. In patients who undergo complete (R0) surgical resection of liver or lung metastases, long-term survival or even a cure is possible. These patients can achieve 5-year survival rates of 20–50%, and in some cases, up to 70%.
In systemic therapy, recent years have brought notable advancements. First-line chemotherapy now includes agents like oxaliplatin, irinotecan, and fluoropyrimidines. These are often combined with targeted treatments such as anti-EGFR monoclonal antibodies (cetuximab, panitumumab) for tumors without KRAS mutations or anti-VEGF therapies (bevacizumab, aflibercept, ramucirumab, or the oral tyrosine kinase inhibitor regorafenib). The integration of these chemotherapy and targeted therapy combinations has extended median overall survival to approximately 40 months.
Find out more about metastatic colorectal cancer drugs @ Metastatic Colorectal Cancer Treatment
A snapshot of the Pipeline Metastatic Colorectal Cancer Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| HLX10 (Serplulimab) + HLX04 | Shanghai Henlius Biotech | III | Programmed cell death-1 receptor antagonists; Vascular endothelial growth factor A inhibitors | Intravenous |
| Lenvatinib | Eisai Inc. | III | Vascular endothelial growth factor receptor (VEGFR) 1–3 inhibitor; fibroblast growth factor receptors 1–4 inhibitor; platelet-derived growth factor receptor alpha, inhibitor | Intravenous |
| PolyPEPI-1018 | Treos Bio | II | Immunostimulants | Subcutaneous |
| AUM-001 | AUM Biosciences | II | MAP kinase kinase 1 inhibitors; MAP kinase kinase 2 inhibitors | Oral |
| DKN-01 | Leap Therapeutics, Inc. | II | DKK1 protein inhibitors | Intravenous |
| RP3 | Replimune | II | Cytotoxic T-lymphocyte antigen 4 inhibitor; Immunologic cytotoxicity; Membrane glycoprotein modulator | Intratumoral |
| E7386 | Eisai | I/II | CBP/β-catenin interaction inhibitor | Oral |
Learn more about the emerging metastatic colorectal cancer therapies @ Metastatic Colorectal Cancer Clinical Trials
Metastatic Colorectal Cancer Therapeutics Assessment
The metastatic colorectal cancer pipeline report proffers an integral view of the emerging metastatic colorectal cancer therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Metastatic Colorectal Cancer Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Platelet-derived growth factor alpha receptor antagonists, Proto-oncogene protein c-kit inhibitors, Proto-oncogene protein c-ret inhibitors, Type 1 fibroblast growth factor receptor antagonists, Type 3 fibroblast growth factor receptor antagonists, Type 4 fibroblast growth factor receptor antagonists, Type-2 fibroblast growth factor receptor antagonists, Vascular endothelial growth factor receptor-1 antagonists, Vascular endothelial growth factor receptor-2 antagonists, Vascular endothelial growth factor receptor-3 antagonists, MAP kinase kinase 1 inhibitors, MAP kinase kinase 2 inhibitors, DKK1 protein inhibitors, Apoptosis stimulants, Cadherin modulators, Receptor protein-tyrosine kinase modulators, Transcription factor modulators, Tubulin polymerisation inhibitors, Cell death stimulants, Cytotoxic T-lymphocyte antigen 4 inhibitors, Immunologic cytotoxicity, Membrane glycoprotein modulators
- Key Metastatic Colorectal Cancer Companies: Shanghai Henlius Biotech, Eisai Inc., Treos Bio, AUM Biosciences, Leap Therapeutics, Inc., Hoffmann-La Roche, Replimune, Qilu Pharmaceutical, Lutris Pharma, Ipsen, Bold Therapeutics, Pacylex Pharmaceuticals, FivepHusion, SystImmune, Inspirna, Inc., Sapience Therapeutics, Daiichi Sankyo, Hutchmed, Merus N.V., and others.
- Key Metastatic Colorectal Cancer Pipeline Therapies: HLX10 (Serplulimab) + HLX04, Lenvatinib, PEPI1018, AUM-001, DKN-01, RO7122290, E7386, RP3, QL 1706, LUT014, Liposomal irinotecan, BOLD-100, Zelenirstat, Deflexifol, SI-B003, RGX202, ST316, Trastuzumab Deruxtecan, Surufatinib, Petosemtamab, and others.
Dive deep into rich insights for new metastatic colorectal cancer treatments, visit @ Metastatic Colorectal Cancer Drugs
Table of Contents
| 1. | Metastatic Colorectal Cancer Pipeline Report Introduction |
| 2. | Metastatic Colorectal Cancer Pipeline Report Executive Summary |
| 3. | Metastatic Colorectal Cancer Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Metastatic Colorectal Cancer Clinical Trial Therapeutics |
| 6. | Metastatic Colorectal Cancer Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Metastatic Colorectal Cancer Pipeline: Late-Stage Products (Phase III) |
| 8. | Metastatic Colorectal Cancer Pipeline: Mid-Stage Products (Phase II) |
| 9. | Metastatic Colorectal Cancer Pipeline: Early-Stage Products (Phase I) |
| 10. | Metastatic Colorectal Cancer Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Metastatic Colorectal Cancer Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Metastatic Colorectal Cancer Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the metastatic colorectal cancer pipeline therapeutics, reach out @ Metastatic Colorectal Cancer Therapeutics
Related Reports
Metastatic Colorectal Cancer Epidemiology Forecast
Metastatic Colorectal Cancer Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted metastatic colorectal cancer epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Metastatic Colorectal Cancer Market
Metastatic Colorectal Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key metastatic colorectal cancer companies, including Johnson & Johnson Innovative Medicine, Shanghai Henlius Biotech, Inspirna, Treos Bio, Cardiff Oncology, Agenus, Leap Therapeutics, Arcus Biosciences, Enterome, Tizona Therapeutics, Innovative Cellular Therapeutics, among others.
Colorectal Cancer Market
Colorectal Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key colorectal cancer companies, including Mirati Therapeutics, Exelixis, Enterome, Arcus Biosciences, Lyell Immunopharma, AstraZeneca, Novartis Pharmaceuticals, Surgimab, Numab Therapeutics, SOTIO Biotech, Amgen, Sichuan Baili Pharmaceutical, Qilu Pharmaceutical, Bristol-Myers Squibb, NGM Biopharmaceuticals, Takeda, PureTech, Pfizer, Kezar Life Sciences, Salubris Biotherapeutics, among others.
Colorectal Cancer Pipeline
Colorectal Cancer Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key colorectal cancer companies, including Exelixis, Mirati Therapeutics, Merck Sharp & Dohme LLC, Daiichi Sankyo Company, Inspirna, Lyell Immunopharma, Genentech, Cantargia AB, Arcus Biosciences, Inc, Neogap Therapeutics AB, Criterium, Inc, Daiichi Sankyo, Inc, Bristol-Myers Squibb, Celyad Oncology SA, Pfizer, Akeso, Menarini Group, Elpiscience (Suzhou) Biopharma, Ltd., BeyondBio Inc., Shanghai Henlius Biotech, Rottapharm Biotech, Innovative Cellular Therapeutics Inc., BioNTech SE, among others.
Colorectal Cancer Epidemiology
Colorectal Cancer Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted colorectal cancer epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Oncology Conference Coverage Services
DelveInsight’s Oncology Conference Coverage Services offer a thorough analysis of outcomes from major events like ASCO, ESMO, ASH, AACR, ASTRO, SOHO, SITC, the European CAR T-cell Meeting, and IASLC. This detailed examination provides businesses with essential insights for competitive intelligence and market trend forecasting, supporting the formulation of future strategies.
Get in touch with us today to learn how we can provide AACR coverage exclusively for you at the AACR Meeting 2025
Other Business Consulting Services
Healthcare Competitive Intelligence
Healthcare Licensing Services
Healthcare Portfolio Management
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn
CONTACT: Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com




