The neurofibromatosis type 1 market remains niche, but awareness campaigns, increased diagnoses, and treatment adoption, along with ongoing clinical trials, will expand the NF1 therapeutics market during the forecast period (2025-2034). Unmet needs, particularly in adult populations and systemic disease management, create significant opportunities for new entrants and differentiated strategies
New York, USA, May 15, 2025 (GLOBE NEWSWIRE) — Neurofibromatosis Type 1 Market Set to Witness Significant Growth During the Study Period (2020–2034) Driven by Advances in Treatment | DelveInsight
The neurofibromatosis type 1 market remains niche, but awareness campaigns, increased diagnoses, and treatment adoption, along with ongoing clinical trials, will expand the NF1 therapeutics market during the forecast period (2025-2034). Unmet needs, particularly in adult populations and systemic disease management, create significant opportunities for new entrants and differentiated strategies.
DelveInsight’s Neurofibromatosis Type 1 Market Insights report includes a comprehensive understanding of current treatment practices, emerging neurofibromatosis type 1 drugs, market share of individual therapies, and current and forecasted neurofibromatosis type 1 market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the Neurofibromatosis Type 1 Market Report
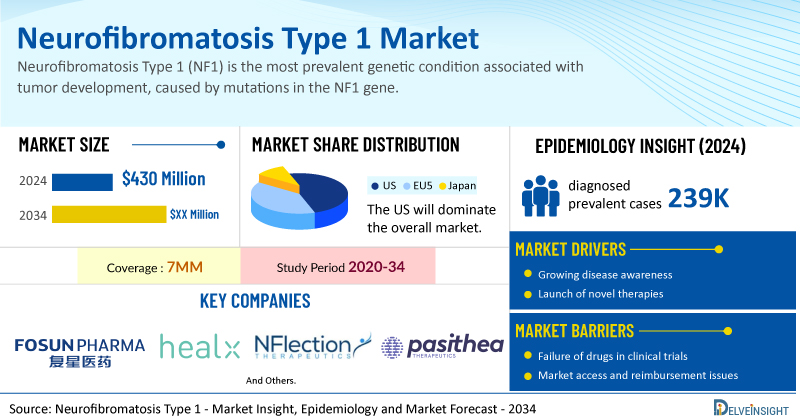
- According to DelveInsight’s analysis, the total market size of NF1 in the 7MM in 2024 was approximately USD 430 million. This is anticipated to grow by 2034, driven by the entry of new emerging therapies.
- Based on DelveInsight’s assessment in 2024, the 7MM had ~239K diagnosed prevalent cases of NF1. These cases are expected to increase by 2034.
- Prominent companies working in the domain of neurofibromatosis type 1, including Fosun Pharmaceutical, Healx, NFlection Therapeutics, Pasithea Therapeutics, and others, are actively working on innovative neurofibromatosis type 1 drugs. These novel neurofibromatosis type 1 therapies are anticipated to enter the neurofibromatosis type 1 market in the forecast period and are expected to change the market.
- Some of the key neurofibromatosis type 1 therapies in the pipeline include FCN-159, HLX-1502, NFX-179, PAS-004, and others.
- In November 2024, AstraZeneca and Merck announced positive topline results from the Phase III KOMET trial in adults with neurofibromatosis type 1 who have symptomatic, inoperable plexiform neurofibromas.
- In October 2024, Healx announced that the US FDA granted FTD to HLX-1502 for the treatment of NF1.
Discover which therapies are expected to grab the neurofibromatosis type 1 market share @ Neurofibromatosis Type 1 Market Report
Neurofibromatosis Type 1 Overview
Neurofibromatosis Type 1 (NF1) is the most prevalent genetic condition associated with tumor development, caused by mutations in the NF1 gene. These mutations lead to a loss of neurofibromin, a protein that normally regulates RAS signaling, resulting in the formation of plexiform neurofibromas—tumors of the peripheral nerve sheath that can significantly affect quality of life. Neurofibromatosis Type 1 is typically diagnosed during early childhood (as NF1-PN) or later in adulthood (as cNF), often based on visible indicators like café-au-lait spots or mild tissue overgrowth.
However, plexiform neurofibromas that develop deep within the body may go unnoticed until symptoms such as pain appear, prompting the need for imaging to confirm their presence. These tumors are particularly challenging due to their tendency to infiltrate nearby tissues, their irregular structure, and high blood vessel density. They frequently occur in sensitive areas such as the head, neck, chest, or spine, making surgical intervention risky and complex.
Early recognition, appropriate imaging, and expert evaluation are key to diagnosis. Effective management requires a multidisciplinary team, including specialists in genetics, neurology, radiology, and surgery, to address both the physical and emotional burden on children and their families.
Neurofibromatosis Type 1 Epidemiology Segmentation
The neurofibromatosis type 1 epidemiology section provides insights into the historical and current neurofibromatosis type 1 patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The neurofibromatosis type 1 market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Diagnosed Prevalent Cases of Neurofibromatosis Type 1
- Diagnosed Prevalent Cases of Neurofibromatosis Type 1 Manifestations
- Age-specific Diagnosed Prevalent Cases of Neurofibromatosis Type 1
- Severity-specific Diagnosed Prevalent Cases of cNFs
- Age-specific Diagnosed Prevalent Cases of Neurofibromatosis Type 1-PN
- Neurofibromatosis Type 1-PN Cases by Clinical Symptoms
- Neurofibromatosis Type 1-PN Cases Eligible for Surgery
Download the report to understand which factors are driving neurofibromatosis type 1 epidemiology trends @ Neurofibromatosis Type 1 Epidemiological Insights
Neurofibromatosis Type 1 Treatment Market
Currently, there is no definitive cure for neurofibromatosis, so care focuses on regular monitoring and addressing complications as they arise. For NF1, treatment is mainly aimed at managing symptoms and related health issues.
KOSELUGO (selumetinib), a kinase inhibitor, received FDA approval in April 2020 for use in children aged 2 and older with neurofibromatosis type 1 who have symptomatic, inoperable plexiform neurofibromas. It represents the first FDA-approved therapy specifically targeting PN, providing a new treatment pathway for this rare and challenging condition.
Data suggests that KOSELUGO’s average launch price in the EU exceeds that of the US (based on weight-adjusted dosing), potentially reflecting differences in payer expectations across regions during pricing negotiations. In the US, KOSELUGO has been introduced under value-based agreements, which may indicate greater uncertainty regarding its cost-effectiveness due to variable dosing requirements.
In February 2025, the FDA approved GOMEKLI (mirdametinib), a MEK inhibitor developed by SpringWorks, for use in adults and children aged 2 and older with neurofibromatosis type 1 who have symptomatic plexiform neurofibromas (PN) that cannot be completely removed surgically. Alongside the approval, SpringWorks received a Priority Review Voucher (PRV) under the FDA’s Rare Pediatric Disease program. GOMEKLI was approved for Priority Review and had previously received Orphan Drug and Fast Track designations for treating NF1-associated PN.
Learn more about the neurofibromatosis type 1 treatment options @ Cure for Neurofibromatosis Type 1
Neurofibromatosis Type 1 Emerging Drugs and Companies
Some of the drugs for NF1 in the pipeline include FCN-159 (Fosun Pharmaceutical), HLX-1502 (Healx), NFX-179 (NFlection Therapeutics), and PAS-004 (Pasithea Therapeutics), among others.
HLX-1502 is an orally administered tablet that functions through a novel mechanism, offering a distinct investigational treatment option for patients with NF1. Healx has received clearance from the US FDA for an IND application, enabling the start of a Phase II clinical trial focused on adults with NF1 and inoperable plexiform neurofibroma.
In October 2024, the US FDA granted Fast Track Designation (FTD) to HLX-1502 for treating NF1. Additionally, in September 2023, HLX-1502 was awarded Orphan Drug Designation (ODD) for the same condition.
NFlection is developing a topical gel formulation of NFX-179, a proprietary “soft” (metabolically labile) MEK inhibitor, designed to treat cutaneous neurofibromas (cNFs) that persistently develop in patients with neurofibromatosis type 1 (NF1). In preclinical studies using an NF1 mouse model, MEK inhibitors significantly reduced neurofibroma tumor volume after two months of treatment.
While an oral MEK inhibitor is currently approved for treating plexiform neurofibromas in NF1 patients, its systemic administration often leads to serious side effects such as severe acneiform rash, gastrointestinal issues, fatigue, edema, hypertension, cardiomyopathy, and retinal detachment. To address this, NFlection created a cosmetically appealing topical version of NFX-179 to minimize systemic exposure while targeting cNFs. When tested on human cNF explants, NFX-179 showed a dose-dependent reduction in phosphorylated ERK (pERK), a key marker of Ras/Raf/MEK/ERK pathway activity.
In a completed Phase 2a clinical trial, the gel effectively reduced pERK levels in cNFs. More recently, NFlection completed a randomized, double-blind, placebo-controlled Phase 2b study with 199 participants, showing that NFX-179 significantly reduced cNF size compared to placebo.
The anticipated launch of these emerging therapies are poised to transform the neurofibromatosis type 1 market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the neurofibromatosis type 1 market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about neurofibromatosis type 1 clinical trials, visit @ Neurofibromatosis Type 1 Treatment Drugs
Neurofibromatosis Type 1 Market Dynamics
The neurofibromatosis type 1 market dynamics are anticipated to change in the coming years. The introduction of MEK inhibitors has transformed the treatment landscape for plexiform neurofibromas, offering significant short-term clinical benefits, particularly in children, by modifying the natural growth progression of these tumors. Additionally, the burden of cutaneous neurofibromas in NF1 patients further emphasizes the need for safe and effective therapies, representing a valuable research and development opportunity in the NF1 market
Furthermore, many potential therapies are being investigated for the treatment of neurofibromatosis type 1, and it is safe to predict that the treatment space will significantly impact the neurofibromatosis type 1 market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the neurofibromatosis type 1 market in the 7MM.
However, several factors may impede the growth of the neurofibromatosis type 1 market. Despite recent efforts to provide recommendations, the absence of definitive consensus guidelines for plexiform neurofibroma surveillance and predictive factors for cutaneous neurofibroma development in NF1 leads to practice variability, while the rise of MEK inhibitors such as FCN-159 may intensify market competition, and accessibility challenges—such as the large capsule size of KOSELUGO for pediatric patients—remain a key limitation.
Moreover, neurofibromatosis type 1 treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the neurofibromatosis type 1 market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the neurofibromatosis type 1 market growth.
| Neurofibromatosis Type 1 Report Metrics | Details |
| Study Period | 2020–2034 |
| Neurofibromatosis Type 1 Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Neurofibromatosis Type 1 Market Size in 2024 | USD 430 Million |
| Key Neurofibromatosis Type 1 Companies | Fosun Pharmaceutical, Healx, NFlection Therapeutics, Pasithea Therapeutics, AstraZeneca, Merck, SpringWorks Therapeutics, and others |
| Key Neurofibromatosis Type 1 Therapies | FCN-159, HLX-1502, NFX-179, PAS-004, KOSELUGO, GOMEKLI, and others |
Scope of the Neurofibromatosis Type 1 Market Report
- Neurofibromatosis Type 1 Therapeutic Assessment: Neurofibromatosis Type 1 current marketed and emerging therapies
- Neurofibromatosis Type 1 Market Dynamics: Conjoint Analysis of Emerging Neurofibromatosis Type 1 Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Neurofibromatosis Type 1 Market Access and Reimbursement
Discover more about neurofibromatosis type 1 drugs in development @ Neurofibromatosis Type 1 Clinical Trials
Table of Contents
| 1 | KEY INSIGHTS |
| 2 | REPORT INTRODUCTION |
| 3 | EXECUTIVE SUMMARY OF NF1 |
| 4 | EPIDEMIOLOGY AND MARKET METHODOLOGY |
| 5 | NF1-PN MARKET OVERVIEW AT A GLANCE |
| 5.1 | MARKET SHARE (%) DISTRIBUTION OF NF1 BY THERAPIES IN 2020 IN THE 7MM |
| 5.2 | MARKET SHARE (%) DISTRIBUTION OF NF1 BY THERAPIES IN 2034 IN THE 7MM |
| 6 | KEY EVENTS |
| 7 | DISEASE BACKGROUND AND OVERVIEW |
| 7.1 | INTRODUCTION |
| 7.2 | GENETIC MUTATIONS |
| 7.3 | CLINICAL MANIFESTATIONS |
| 7.4 | CAUSES |
| 7.5 | DIAGNOSIS |
| 7.5.1 | Differential Diagnosis |
| 7.5.2 | Diagnostic Algorithm |
| 7.5.3 | Diagnostic Guidelines |
| 7.6 | TREATMENT AND MANAGEMENT |
| 7.6.1 | Treatment Algorithm |
| 8 | EPIDEMIOLOGY AND PATIENT POPULATION |
| 8.1 | KEY FINDINGS |
| 8.2 | ASSUMPTION AND RATIONALE |
| 8.3 | TOTAL DIAGNOSED PREVALENT CASES OF NF1 IN THE 7MM |
| 8.4 | THE UNITED STATES |
| 8.4.1 | Total Diagnosed Prevalent Cases of NF1 in the United States |
| 8.4.2 | Diagnosed Prevalent Cases of NF1 Manifestations in the United States |
| 8.4.3 | Age-specific Diagnosed Prevalent Cases of NF1 in the United States |
| 8.4.4 | Severity-specific Diagnosed Prevalent Cases of cNFs in the United States |
| 8.4.5 | Age-specific Diagnosed Prevalent Cases of NF1-PN in the United States |
| 8.4.6 | NF1-PN Cases by Clinical Symptoms in the United States |
| 8.4.7 | NF1-PN Cases Eligible for Surgery in the United States |
| 8.5 | EU4 AND THE UK |
| 8.5.1 | Total Diagnosed Prevalent Cases of NF1 in EU4 and the UK |
| 8.5.2 | Diagnosed Prevalent Cases of NF1 Manifestations in EU4 and the UK |
| 8.5.3 | Age-specific Diagnosed Prevalent Cases of NF1-PN in EU4 and the UK |
| 8.5.4 | Severity-specific Diagnosed Prevalent Cases of cNFs in EU4 and the UK |
| 8.5.5 | Age-specific Diagnosed Prevalent Cases of NF1-PN in EU4 and the UK |
| 8.5.6 | NF1-PN Cases by Clinical Symptoms in EU4 and the UK |
| 8.5.7 | NF1-PN Cases Eligible for Surgery in EU4 and the UK |
| 8.6 | JAPAN |
| 8.6.1 | Total Diagnosed Prevalent Cases of NF1 in Japan |
| 8.6.2 | Diagnosed Prevalent Cases of NF1 Manifestations in Japan |
| 8.6.3 | Age-specific Diagnosed Prevalent Cases of NF1-PN in Japan |
| 8.6.4 | Severity-specific Diagnosed Prevalent Cases of cNFs in Japan |
| 8.6.5 | Age-specific Diagnosed Prevalent Cases of NF1-PN in Japan |
| 8.6.6 | NF1-PN Cases by Clinical Symptoms in Japan |
| 8.6.7 | NF1-PN Cases Eligible for Surgery in Japan |
| 9 | PATIENT JOURNEY |
| 10 | MARKETED DRUGS |
| 10.1 | KEY CROSS COMPETITION |
| 10.2 | KOSELUGO (SELUMETINIB): ASTRAZENECA AND MERCK |
| 10.2.1 | Product Description |
| 10.2.2 | Regulatory Milestones |
| 10.2.3 | Other Development Activities |
| 10.2.4 | Clinical Development |
| 10.2.5 | Safety and Efficacy |
| 11 | EMERGING DRUGS |
| 11.1 | KEY CROSS COMPETITION |
| 11.2 | MIRDAMETINIB (PD-0325901): SPRINGWORKS THERAPEUTICS |
| 11.2.1 | Product Description |
| 11.2.2 | Other Developmental Activities |
| 11.2.3 | Clinical Development |
| 11.2.4 | Safety and Efficacy |
| 11.3 | FCN-159: FOSUN PHARMACEUTICAL |
| 11.3.1 | Product Description |
| 11.3.2 | Clinical Development |
| 11.3.3 | Safety and Efficacy |
| 11.4 | HLX-1502: HEALX |
| 11.4.1 | Product Description |
| 11.4.2 | Other Developmental Activities |
| 11.4.3 | Clinical Development |
| 11.5 | NFX‑179: NFlection Therapeutics |
| 11.5.1 | Product Description |
| 11.5.2 | Clinical Development |
| 11.5.3 | Safety and Efficacy |
| 12 | NF1-PN: SEVEN MAJOR MARKET ANALYSIS |
| 12.1 | KEY FINDINGS |
| 12.2 | MARKET OUTLOOK |
| 12.3 | CONJOINT ANALYSIS |
| 12.4 | KEY MARKET FORECAST ASSUMPTIONS |
| 12.4.1 | Cost Assumptions and Rebates |
| 12.4.2 | Pricing Trends |
| 12.4.3 | Analogue Assessment |
| 12.4.4 | Launch Year and Therapy Uptakes |
| 12.5 | TOTAL MARKET SIZE OF NF1 IN THE 7MM |
| 12.6 | THE UNITED STATES |
| 12.6.1 | Total Market Size of NF1 in the US |
| 12.6.2 | Market Size of NF1 by Therapies in the US |
| 12.7 | EU4 AND THE UK |
| 12.7.1 | Total Market Size of NF1 in the EU4 and the UK |
| 12.7.2 | Market Size of NF1 by Therapies in EU4 and the UK |
| 12.8 | JAPAN |
| 12.8.1 | Total Market Size of NF1 in Japan |
| 12.8.2 | Market Size of NF1 by Therapies in Japan |
| 13 | UNMET NEEDS |
| 14 | SWOT ANALYSIS |
| 15 | KOL VIEWS |
| 16 | MARKET ACCESS AND REIMBURSEMENT |
| 16.1 | UNITED STATES |
| 16.1.1 | Centre for Medicare and Medicaid Services (CMS) |
| 16.2 | EU4 AND THE UK |
| 16.2.1 | Germany |
| 16.2.2 | France |
| 16.2.3 | Italy |
| 16.2.4 | Spain |
| 16.2.5 | United Kingdom |
| 16.3 | JAPAN |
| 16.3.1 | MHLW |
| 16.4 | MARKET ACCESS AND REIMBURSEMENT OF NF1 |
| 17 | APPENDIX |
| 17.1 | BIBLIOGRAPHY |
| 17.2 | REPORT METHODOLOGY |
| 18 | DELVEINSIGHT CAPABILITIES |
| 19 | DISCLAIMER |
| 20 | ABOUT DELVEINSIGHT |
Related Reports
Neurofibromatosis Type 1 Pipeline
Neurofibromatosis Type 1 Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Neurofibromatosis Type 1 companies, including Shanghai Fosun Pharmaceutical, NFlection Therapeutics, Pasithea Therapeutics Corp., Novartis, Pfizer, among others.
Neurofibromatosis Type 1 Epidemiology
Neurofibromatosis Type 1 Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, and the neurofibromatosis type 1 epidemiology trends.
Neurofibromatosis Type 2 Market
Neurofibromatosis Type 2 Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key neurofibromatosis type 2 companies including Recursion Pharmaceuticals, Takeda, Vivace Therapeutics, AstraZeneca, Recursion Pharmaceuticals Inc., Betta Pharmaceuticals Co. Ltd., Shandong Simcere-Medgenn Bio-pharmaceutical Co. Ltd, Novartis Pharmaceuticals, Genentech Inc., GlaxoSmithKline, AstraZeneca, among others.
Neurofibromatosis Type 2 Pipeline
Neurofibromatosis Type 2 Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key neurofibromatosis type 2 companies, including AstraZeneca, Recursion Pharmaceuticals, Betta Pharmaceuticals Co., Ltd., Novartis Pharmaceuticals, Genentech, Takeda, among others.
Neurofibromatosis Type 1-associated Plexiform Neurofibromas Market
Neurofibromatosis Type 1-associated Plexiform Neurofibromas Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key NF1-PN companies including AstraZeneca, Merck, SpringWorks Therapeutics, Healx, Fosun Pharmaceutical, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Pipeline Assessment
Healthcare Licensing Services
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
CONTACT: Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com




